CareSens Air App
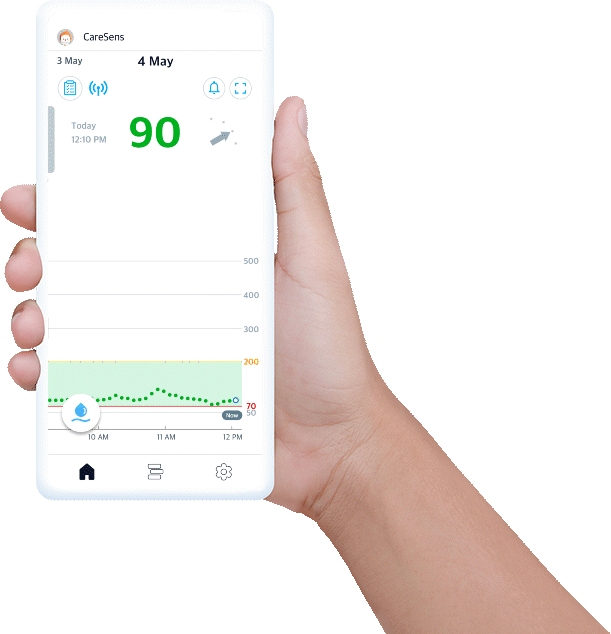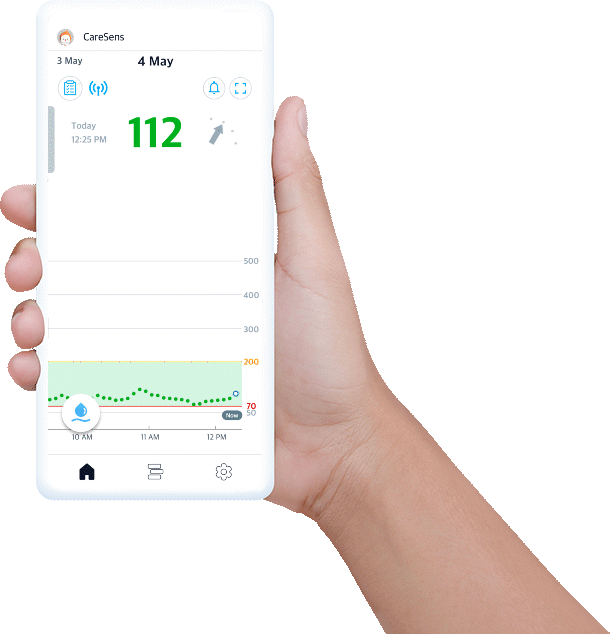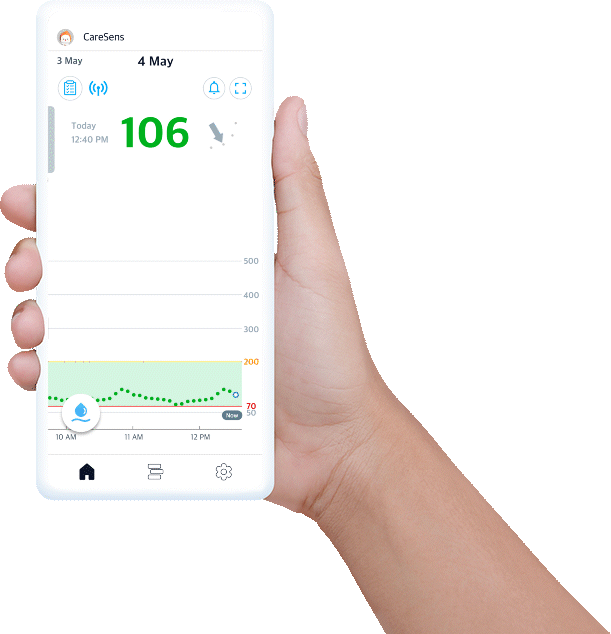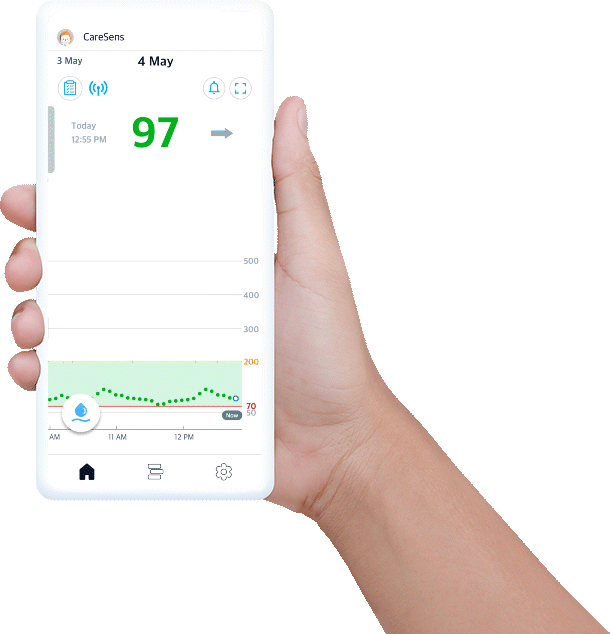
Experience easy glucose management and data sharing with CareSens Air app.
With the CareSens Air application:
- Displays your real-time glucose trends and life events at once.
- Provides glucose statistics for better understanding.
- Set alerts to help you reach management target levels.
* The actual app screen may differ depending on the software version.
* Smart devices are not included in the product.